As wind and solar power becomes a larger proportion of the world’s energy supply, methods for smoothing their unreliable output become more important. Long-term energy storage is key; buying cheap electricity during peaks in supply to then sell back to the grid when demand is higher.
Hydrogen has the potential fill this role, since it embodies a very high amount of energy per kilogram, and it can be processed without emission of carbon dioxide or other pollutants. To implement hydrogen energy storage, there are three stages: production, storage and conversion to electricity.
The most common method of producing hydrogen is through a process called steam-methane reforming. This chemical reaction sees water and methane converted to hydrogen and carbon dioxide at high temperature. It is cheap, reliable and easy to perform at large scale. However, since it produces carbon dioxide as a by-product, it defeats the purpose of renewable energy generation in the first place. The hydrogen is also quite low purity and requires expensive refining if it is to be used in fuel cells later on.

A hydrogen car refuellling in just a few minutes
The more environmentally friendly option is electrolysis. Here, water is broken down by a large voltage into hydrogen and oxygen, with no greenhouse-gas emissions as by-products. The three primary methods of electrolysis are alkaline water electrolysis (AWE), polymer electrolyte membrane (PEM) electrolysis and solid-oxide electrolysis (SOE). Their relative advantages and disadvantages are shown in the table below, but the fundamental result is the same: a clean source of high-purity hydrogen. The trade-off is a relatively higher cost compared with SMR and safety issues that could arise should the hydrogen and oxygen ever mix.
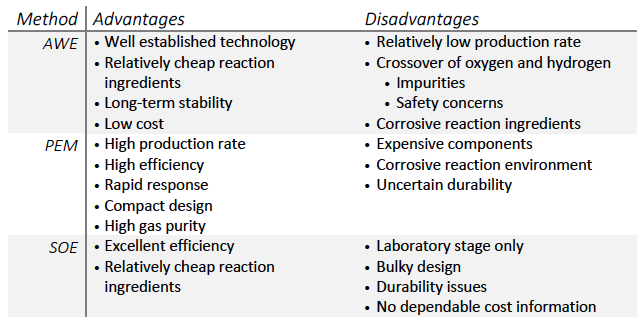
Table comparing the relative advantages and disadvantages of various methods of hydrogen production by electrolysis of water. Adapted from [1]
On a large scale, liquid hydrogen storage is currently the best solution where geography is not conducive to underground storage. However, it is more costly than simple compression and hydrogen is unavoidably lost in the process.
There are more exotic methods available that still require some development. It is possible to absorb the hydrogen into microporous materials such as carbon nanotubes, but that doesn’t solve the density problem and is mostly being considered for hydrogen-powered cars. Reacting the hydrogen into new chemical compounds that easily release it when required is more promising density-wise but would have a slower response time and is further back in development still.
The hydrogen is not stored forever. There are two main ways to convert hydrogen into electricity: combustion or fuel cells. Combustion would work in the same way as a natural gas power station, which is its main attraction. It is possible to retrofit existing stations, and the operating parameters – such as peak power output or response time – are well characterised.
Response time is where fuel cells shine. Their response times are almost as good as battery banks, which would be vital for smoothing out short-term fluctuations in power that may occur due to, for example, partially cloudy days over a solar farm. Unlike combustion engines, their efficiency doesn’t tail off as they reach peak output. However, maximum power output is only around 1 MW for a single fuel cell, requiring many cells to be coupled together, increasing costs.
The two methods are close in efficiency for the most modern models: around 60 per cent for gas turbines and 64 per cent for fuel cells. Both methods also benefit from the decoupling of storage capacity and power output. Batteries both store and output the power, so the two factors are closely linked, whereas hydrogen storage can be increased without having to alter the parts that produce the electricity. This allows upgrading the facilities to be more flexible and cheaper.
This speaks to one of the primary downsides, however. The increased number of steps decreases the round-trip efficiency of hydrogen storage. Therefore, it will only be economical to implement if the variation in cost of electricity due to intermittent supply is large. Compared to other methods of storage, however, hydrogen is an attractive prospect as research into the technology promises the possibility of large efficiency gains. It has the potential to deliver high energy density and quick response times without polluting the atmosphere, all key attributes for implementing renewable energy in the future.
[1] Carmo, M., Fritz, D., Mergel, J., & Stolten, D. (2013). A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 38(12), 4901-4934